Dietary supplementation of consortium of probiotics as an effective alternative strategy for replacement for in-feed antibiotic growth promoters
Dr. Sumon Nag Chowdhury, Sr. Technical Manager
Dr. Parshuram Deokar, Product Manager
GLAMAC INTRNATIONAL PVT LTD, India
INTRODUCTION:
A modern challenges in the poultry production is to exploit the use of specific dietary supplements to boost the intrinsic potential of poultry bird to perform better. Antibiotic growth promoters have undoubtedly improved animal performance and health status. It is apparent that antibiotics function by modifying the intestinal microflora. The microbes can develop resistance to these antibiotics and when transferred to human beings, may pose a problem because of the resistance to these antibiotics. This has attracted global attention. Many antibiotics in livestock and poultry production as growth promoters are banned in several countries. Following the ban on the use of antibiotics as growth promoters in animal nutrition by the European Union (EU) in 2006, the nutritionist and researchers are attempting other alternatives claiming to enhance the performance of broiler. The most effective and sustainable solution is the use of consortium of probiotic bacteria. As far FAO and WHO definition, probiotics are “live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host”.
Recently there was a study in eastern part of India in order to evaluate the effects of a consortium of probiotics, ClosBo®, comprising a combination of micro-encapsulated fore gut and hind gut acting proprietary strains of Bacillus subtilis, Bacillus licheniformis, Clostridium butyricum on performance, histo-morphology of jejunum and microbiology of the pooled small intestinal digesta in commercial broiler chickens.
MATERIALS AND METHODS:
A flock of 280 male Cobb 400Y broiler chickens (initial mean body weight 44.8 g) were distributed following a completely randomized block design into 4 treatment groups, each consisting of 7 replicate pens and there were 10 chicks in each pen (n = 70 in a group). The control group diet was formulated close to industry standard with corn, soybean meal and meat-bone meal without any protease or carbohydrate degrading enzymes, whereas three treatment groups respectively contained BMD (50 ppm equivalent to 500 mg/kg diet, ClosBo® at the rate of 200 mg/kg diet and Bacillus subtilis PB6 at the rate of 200 mg/kg diet. The birds were vaccinated against Newcastle disease (5d and 20d) and infectious bursal disease (12d). Diet and drinking water were offered ad libitum.The birds were fed with a starter (1-21 d), and a grower-finisher (22-35 d) diet all prepared fresh at the beginning of each feeding period with raw materials of same lot. All diets were formulated following the ideal protein ratio using standardized ileal digestible amino acid requirement of the birds. Zootechnical parameters like average daily body weight gain (ADG), feed intake, mortality and feed conversion ratio (FCR) were measured weekly. At 35 d one bird was randomly selected from each of the pens (7 chickens per treatment) to evaluate the effects of different dietary treatments on the histo-morphology of the gut.
RESULTS AND DISCUSSION:
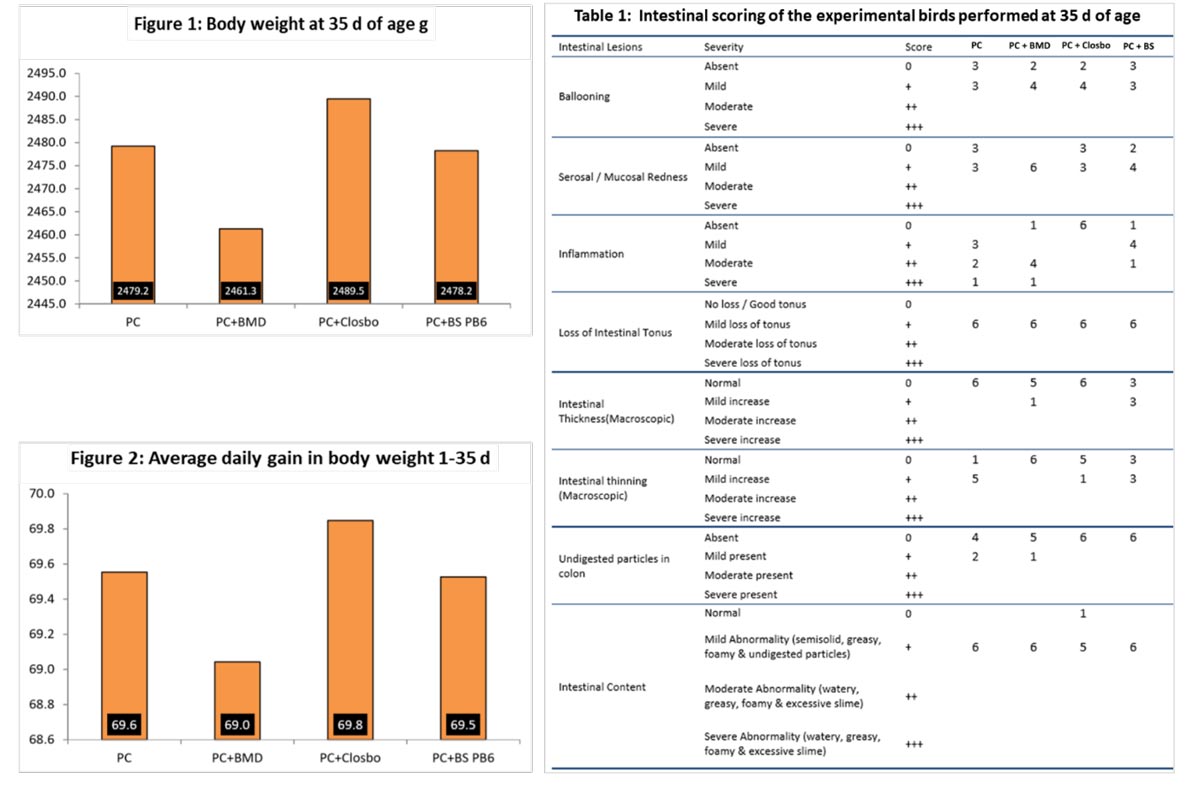
Dietary supplementation of ClosBo® resulted in better average daily gain and almost 30 g more body weight compared to that in the BMD supplemented group at the time of harvest which is substantial in markets where body weight of chickens is the main factor in terms of profit generation. Moreover, the results should be interpreted in the light of a situation when antibiotic growth promoters are to be withdrawn mandatorily from the feeding regimens of broiler chickens with general management practices remaining mostly unchanged.The current results indicate that under that situation dietary supplementation of consortium of probiotics supplements like ClosBo® should be a pragmatic and prudent tool to elicit better body weight responses under similar feeding and management conditions.

Villus height and crypt depth of the jejunum was similar across the groups indicating non-significant effects of the dietary treatments. However, the small intestine of the ClosBo® supplemented group had practically no undigested feed particles as could be observed at the time of intestinal scoring at 35 d of age. Visible inflammation in gut was altogether absent in the group supplemented with ClosBo® while mild to moderate inflammation was present in all the other dietary groups including that supplemented with Bacillus subtilis PB6.
When looked more closely, it was observed that the villi in the PC and the BMD supplemented groups were comparatively shorter and stumpy in appearance and were sparse in distribution while those in the ClosBo® and Bacillus subtilis PB6 supplemented groups were comparatively longer and arranged more closely. Moreover, the structural arrangement of the villi in the ClosBo® supplemented group appeared to be more compact and uniform amongst all the diets. As a matter of fact, this observation corroborates the intestinal scoring which clearly suggested that the small intestinal mucosa was in a comparatively better state of health in the birds which were supplemented with ClosBo®.
CONCLUSIONS:
It was concluded from the present study that dietary supplementation of ClosBo® resulted in better body weight compared to other groups. This may be related to the effect of the dietary treatments on histo-morphology of the jejunum indicating a better disposition with ClosBo® and visually the villi structure and arrangements were better in the ClosBo® supplemented group corroborating the intestinal lesion scoring of the birds which revealed a better status of the small intestinal mucosa. Overall it was concluded that dietary supplementation of ClosBo® may be used as an effective alternative to antibiotic growth promoters like BMD in the feeding regimens of commercial broiler chickens.
